


Next: Calibration and tuning
Up: A HIGH SPECTRAL RESOLUTION
Previous: Data system
Return to the Publications.
Return to the Index.
In order to overcome the limited capability of the high resolution etalon
to separate between aerosol and molecular scattering and to
increase the system stability and reliability, an iodine absorption
filter was constructed.
For the first HSRL measurements a 43 cm long cell was made. The cell
was made from glass tubing with an attached side arm. Optical
quality end windows with anti-reflection coatings were epoxed
to the ends.
The cell
with iodine crystals in a side arm was evacuated and kept at 27  C.
Transferring of the iodine from the absorption cell into the vacuum
pump was prevented by evacuating the cell through a cold trap and cooling
the side arm with liquid nitrogen.
Although the iodine cell can be operated at room temperature,
the operating temperature of the cell has to be controlled,
because the vapor pressure of iodine is very temperature
sensitive [32]. In the HSRL, the cell temperature is maintained
with
C.
Transferring of the iodine from the absorption cell into the vacuum
pump was prevented by evacuating the cell through a cold trap and cooling
the side arm with liquid nitrogen.
Although the iodine cell can be operated at room temperature,
the operating temperature of the cell has to be controlled,
because the vapor pressure of iodine is very temperature
sensitive [32]. In the HSRL, the cell temperature is maintained
with  0.1
0.1  C accuracy by
operating the cell in temperature controlled environment.
C accuracy by
operating the cell in temperature controlled environment.
The iodine spectrum is measured by scanning the laser wavelength
by changing the temperature of the seedlaser under computer control.
A small amount of laser light is directed into a 100 m long fiber optic delay
(Fiber 1 in Figure 4)
and sent to the receiver to create a calibration light source.
The temperature-wavelength dependence of the scan was determined by using the
free spectral range of the high resolution etalon as a reference.
This could be made, because the free spectral range of the etalon
can be calculated when the length of the etalon spacers is known
and the spacing of two (or more) etalon transmission peaks in temperature
units can be measured.
The calibration was made
by simultaneously
measuring the transmission spectrum of the high resolution etalon and
iodine absorption filter.
The simultaneous
measurement of the high resolution etalon and iodine absorption
filter transmissions was made by measuring
the
signal reflected from the high resolution etalon (Figure 12)
and the signal transmitted through the absorption cell. The pressure
in etalon was held constant while the laser wavelength was scanned.
The spectrum was normalized by measuring the cell transmittance without
the iodine cell.
A part of the measured iodine spectrum is presented in Figure 13.
The measured spectrum was compared with a published spectrum
[18] and
an  0.01 pm wavelength agreement in relative line
positions was observed. The linearity of the temperature scan
was confirmed from the free spectral range information of
high resolution etalon by performing the scan over more than one
free spectral range. Single mode operation between two seedlaser
mode hops can be maintained over 20 GHz range (at 1064 nm)
and within this range two high resolution etalon free spectral
ranges can be covered. During a mode hop the laser frequency jumps
back about 10 GHz.
0.01 pm wavelength agreement in relative line
positions was observed. The linearity of the temperature scan
was confirmed from the free spectral range information of
high resolution etalon by performing the scan over more than one
free spectral range. Single mode operation between two seedlaser
mode hops can be maintained over 20 GHz range (at 1064 nm)
and within this range two high resolution etalon free spectral
ranges can be covered. During a mode hop the laser frequency jumps
back about 10 GHz.
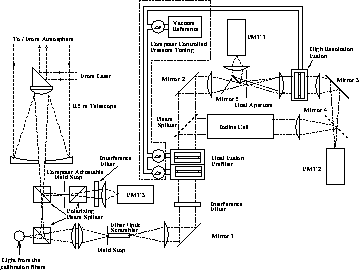
Figure 12: The HSRL receiver used for iodine spectrum calibrations.
The same receiver setup was used for the first HSRL measurements
with the iodine absorption filter. For data taking the transmission of the
high resolution etalon was tuned out from the peak and
the etalon was used as a reflector. When the beamsplitter and
the mirror 4 are removed from the system, the system returns
back to the old HSRL receiver.
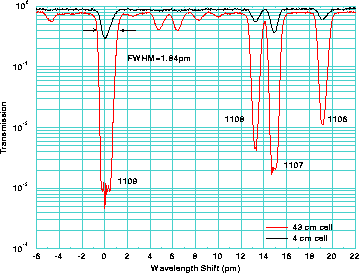
Figure 13: Transmission of a 4 cm and 43 cm iodine cells as a function of
wavelength shift. The identification line numbers are from
Gerstenkorn and Luc .
.
For initial HSRL measurements the line 1109
(peak wavelength 532.26 nm)[18], which is
well isolated from the neighboring lines,
was chosen. The full width half maximum width of the line is  1.8 pm
and the peak transmission is
1.8 pm
and the peak transmission is  0.08%.
The hyperfine structure of the peak 1109 defines the asymmetric shape
of the absorption peak [33]. In fact, the line 1109 is a combination of two
rotational vibrational transitions with different hyperfine structures.
0.08%.
The hyperfine structure of the peak 1109 defines the asymmetric shape
of the absorption peak [33]. In fact, the line 1109 is a combination of two
rotational vibrational transitions with different hyperfine structures.
The iodine absorption cell provides a robust filter for the HSRL, because
it is not dependent on the mechanical alignment of the filter
or the angular dependence of the incoming light. Another advantage
is the stability of the absorption characteristics.
This provides a stable long term operation. The strength of
observed absorption line is dependent on the line strength, and
the length, temperature, and pressure of the cell. By controlling
the operating environment and with a nearly
leak proof system, the current iodine cell is operated for several
months without any maintenance. During this time, a small change
in absorption strength and line width were observed
due to a small leak that
was caused by the iodine penetrating through a hose.
Also the iodine was found
to condense into the walls of the cell, but
even during a long period of time, the amount of condensation
has been small and  10% extra absorption is observed.
The condensation can be prevented by operating the tip of the side arm
couple degrees below the cell temperature. The problems with
reactive iodine penetrating through the hoses can be prevented
by using a sealed all-glass cell. In a short term operation, the stability
of the absorption characteristics has proven to be
so good that a system calibration scans from different days
can be used for the calculations of the system calibration coefficients.
This requires, that the alignment of the receiver
optics is stable.
10% extra absorption is observed.
The condensation can be prevented by operating the tip of the side arm
couple degrees below the cell temperature. The problems with
reactive iodine penetrating through the hoses can be prevented
by using a sealed all-glass cell. In a short term operation, the stability
of the absorption characteristics has proven to be
so good that a system calibration scans from different days
can be used for the calculations of the system calibration coefficients.
This requires, that the alignment of the receiver
optics is stable.
An absorption filter offers a high rejection against aerosol scattering
and therefore it makes the separation between aerosol and
molecular scattering easier.
Also, a wide dynamic range in rejection
against aerosol scattering is achieved by simply changing the
vapor pressure or the length of the cell.
Comparison between high resolution etalon and iodine absorption
filter performance is presented in Figure 14.
A 2:1 separation between molecular and aerosol scattering
by the etalon (Figure 14.b) is measured compared
to a 1000:1 separation in the iodine cell when
operated at 27  C (Figure 14.a). The molecular transmission in Figure
14.a and
Figure 14.b is calculated by using the
Doppler-broadened molecular spectrum at -65
C (Figure 14.a). The molecular transmission in Figure
14.a and
Figure 14.b is calculated by using the
Doppler-broadened molecular spectrum at -65  C. This temperature
is close to the lowest temperature measured
at the tropopause and this gives the smallest transmission through
the iodine absorption cell.
The molecular transmission of the high resolution etalon
and the iodine absorption filter are similar (Figure 14.c).
Due to wide absorption line width,
the molecular
transmission
of the iodine filter is more dependent on the air temperature than the
etalon.
The temperature dependence of the cell transmission is modeled by
using the table values of iodine vapor pressure [32]
(Figure 14.d).
Calculations show, that
by changing the cell temperature from 27
C. This temperature
is close to the lowest temperature measured
at the tropopause and this gives the smallest transmission through
the iodine absorption cell.
The molecular transmission of the high resolution etalon
and the iodine absorption filter are similar (Figure 14.c).
Due to wide absorption line width,
the molecular
transmission
of the iodine filter is more dependent on the air temperature than the
etalon.
The temperature dependence of the cell transmission is modeled by
using the table values of iodine vapor pressure [32]
(Figure 14.d).
Calculations show, that
by changing the cell temperature from 27  C to 0
C to 0  C,
the online transmission can be tuned from 0.08% to 60%.
C,
the online transmission can be tuned from 0.08% to 60%.

Figure 14:
(a) Transmission of 43 cm cell (solid line) together with the molecular
transmission (dashed line) at -65  C air temperature as
a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65
C air temperature as
a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65  C.
(b) Etalon transmission (solid line) and calculated molecular transmission
(dashed line) as a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65
C.
(b) Etalon transmission (solid line) and calculated molecular transmission
(dashed line) as a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65  C.
(c) Comparison of molecular transmission of high resolution etalon
and iodine cell
as a function of air temperature.
(d) Iodine cell aerosol and molecular
transmission as a function of cell temperature.
C.
(c) Comparison of molecular transmission of high resolution etalon
and iodine cell
as a function of air temperature.
(d) Iodine cell aerosol and molecular
transmission as a function of cell temperature.



Next: Calibration and tuning
Up: A HIGH SPECTRAL RESOLUTION
Previous: Data system
Return to the Publications.
Return to the Index.
Paivi Piironen
Tue Mar 26 20:49:55 CST 1996
 C.
Transferring of the iodine from the absorption cell into the vacuum
pump was prevented by evacuating the cell through a cold trap and cooling
the side arm with liquid nitrogen.
Although the iodine cell can be operated at room temperature,
the operating temperature of the cell has to be controlled,
because the vapor pressure of iodine is very temperature
sensitive [32]. In the HSRL, the cell temperature is maintained
with
C.
Transferring of the iodine from the absorption cell into the vacuum
pump was prevented by evacuating the cell through a cold trap and cooling
the side arm with liquid nitrogen.
Although the iodine cell can be operated at room temperature,
the operating temperature of the cell has to be controlled,
because the vapor pressure of iodine is very temperature
sensitive [32]. In the HSRL, the cell temperature is maintained
with  0.1
0.1  C accuracy by
operating the cell in temperature controlled environment.
C accuracy by
operating the cell in temperature controlled environment.



 0.01 pm wavelength agreement in relative line
positions was observed. The linearity of the temperature scan
was confirmed from the free spectral range information of
high resolution etalon by performing the scan over more than one
free spectral range. Single mode operation between two seedlaser
mode hops can be maintained over 20 GHz range (at 1064 nm)
and within this range two high resolution etalon free spectral
ranges can be covered. During a mode hop the laser frequency jumps
back about 10 GHz.
0.01 pm wavelength agreement in relative line
positions was observed. The linearity of the temperature scan
was confirmed from the free spectral range information of
high resolution etalon by performing the scan over more than one
free spectral range. Single mode operation between two seedlaser
mode hops can be maintained over 20 GHz range (at 1064 nm)
and within this range two high resolution etalon free spectral
ranges can be covered. During a mode hop the laser frequency jumps
back about 10 GHz.


 .
.
 1.8 pm
and the peak transmission is
1.8 pm
and the peak transmission is 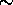 0.08%.
The hyperfine structure of the peak 1109 defines the asymmetric shape
of the absorption peak [
0.08%.
The hyperfine structure of the peak 1109 defines the asymmetric shape
of the absorption peak [ 10% extra absorption is observed.
The condensation can be prevented by operating the tip of the side arm
couple degrees below the cell temperature. The problems with
reactive iodine penetrating through the hoses can be prevented
by using a sealed all-glass cell. In a short term operation, the stability
of the absorption characteristics has proven to be
so good that a system calibration scans from different days
can be used for the calculations of the system calibration coefficients.
This requires, that the alignment of the receiver
optics is stable.
10% extra absorption is observed.
The condensation can be prevented by operating the tip of the side arm
couple degrees below the cell temperature. The problems with
reactive iodine penetrating through the hoses can be prevented
by using a sealed all-glass cell. In a short term operation, the stability
of the absorption characteristics has proven to be
so good that a system calibration scans from different days
can be used for the calculations of the system calibration coefficients.
This requires, that the alignment of the receiver
optics is stable.
 C (Figure
C (Figure  C. This temperature
is close to the lowest temperature measured
at the tropopause and this gives the smallest transmission through
the iodine absorption cell.
The molecular transmission of the high resolution etalon
and the iodine absorption filter are similar (Figure
C. This temperature
is close to the lowest temperature measured
at the tropopause and this gives the smallest transmission through
the iodine absorption cell.
The molecular transmission of the high resolution etalon
and the iodine absorption filter are similar (Figure  C to 0
C to 0  C,
the online transmission can be tuned from 0.08% to 60%.
C,
the online transmission can be tuned from 0.08% to 60%.

 C air temperature as
a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65
C air temperature as
a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65  C.
(b) Etalon transmission (solid line) and calculated molecular transmission
(dashed line) as a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65
C.
(b) Etalon transmission (solid line) and calculated molecular transmission
(dashed line) as a function of wavelength shift.
Dot-dashed line shows the calculated
molecular spectrum at -65  C.
(c) Comparison of molecular transmission of high resolution etalon
and iodine cell
as a function of air temperature.
(d) Iodine cell aerosol and molecular
transmission as a function of cell temperature.
C.
(c) Comparison of molecular transmission of high resolution etalon
and iodine cell
as a function of air temperature.
(d) Iodine cell aerosol and molecular
transmission as a function of cell temperature.